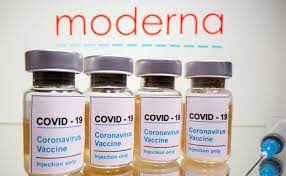
Government gives nod for Cipla to import Moderna’s Covid-19 vaccine
With the Indian Government’s permission to allow Cipla to import Moderna’s Covid-19 vaccine, it became the fourth vaccine in the country to be given the Emergency Use Authorisation (EUA).
India’s drug controller, the Drugs Controller General of India (DCGI) allowed pharmaceutical company Cipla to import Moderna’s Covid-19 vaccine on June 29, 2021.
VK Paul, NITI Aayog’s member (Health), announced this at a press conference of the Health Ministry and said, “The finer modalities are being worked out for the import of the vaccine and added that India is also in conversation with Pfizer and JJ to add to the basket of vaccines available in India.”
In the current basket India has three Covid vaccines — Covaxin, Covishield and Sputnik.
“Moderna will be brought in as a ready-to-use injectable vaccine which can be stored for a period of seven months at prescribed temperature and that normal storage after vial is opened is 30 days,” Dr Paul added.
“The finer modalities are being worked out for the import of the vaccine and added that India is also in conversation with Pfizer and JJ to add to the basket of vaccines available in India.”
-VK Paul, NITI Aayog Member (Health)
Moderna vaccine’s efficacy, according to the World Health Organisation (WHO), is very high at approximately 94.1 per cent.
“Moderna vaccine has shown to have an efficacy of approximately 94.1 per cent in protecting against Covid-19, starting 14 days after the first dose.”
“Based on evidence collected so far, the new variants of SARS-CoV-2, do not alter the effectiveness of the Moderna vaccine,” the WHO added.