Currently, India has four vaccines for emergency use. They are: Covishield, Covaxin, Sputnik-V, and Moderna
PRAVASISAMWAD.COM
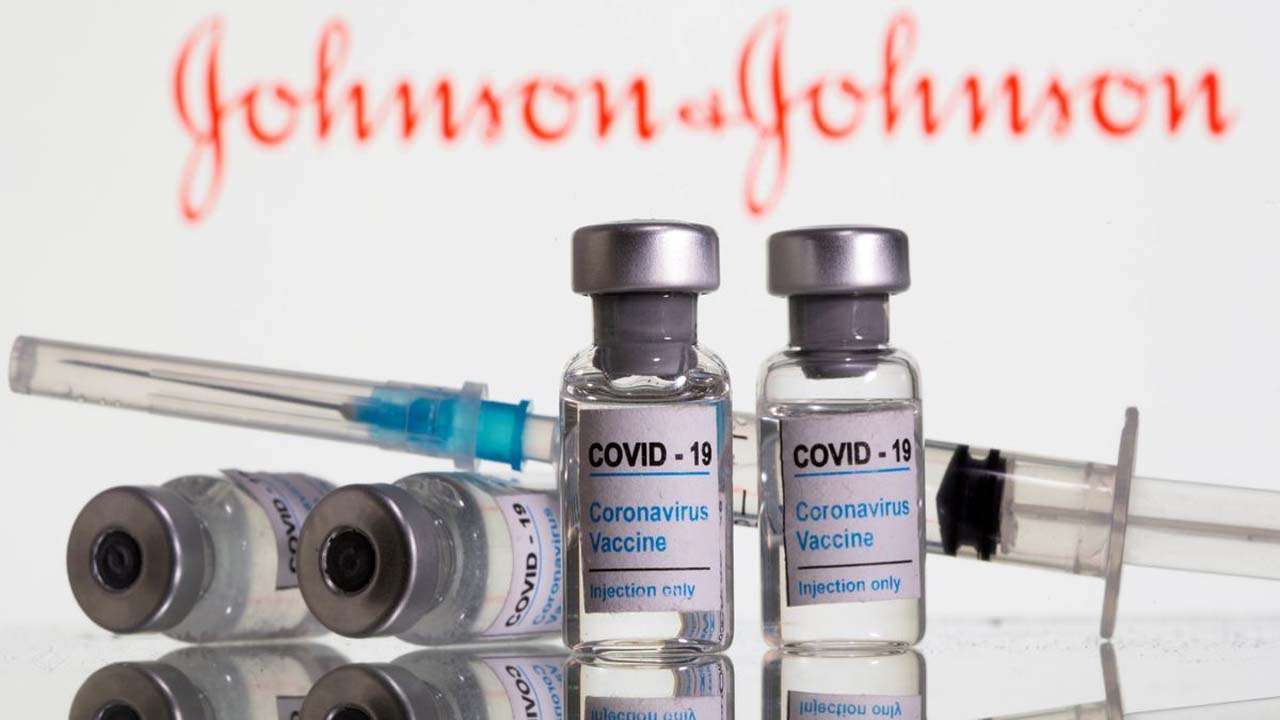
After including US pharma giant Johnson & Johnson’s ‘Janssen’ for emergency usage, India expanded its vaccine basket to five vaccines.
Johnson & Johnson had applied for authorisation of its single-dose Covid-19 vaccine ‘Janssen’ to the Government of India.
Indian Union Health Minister Mansukh Mandaviya tweeted about this development and said, “India expands its vaccine basket! Johnson and Johnson’s single-dose Covid-19 vaccine is given approval for emergency use in India. Now India has 5 EUA vaccines. This will further boost our nation’s collective fight against Covid-19.”
— Union Health Minister Mansukh Mandaviya
Currently, India has four vaccines for emergency use. They are: Covishield, Covaxin, Sputnik-V, and Moderna.
Indian Union Health Minister Mansukh Mandaviya tweeted about this development and said, “India expands its vaccine basket! Johnson and Johnson’s single-dose Covid-19 vaccine is given approval for emergency use in India. Now India has 5 EUA vaccines. This will further boost our nation’s collective fight against Covid-19.”
It is too early to say anything about Janssen’s roll out in the Indian market, but whenever it comes, the single-dose vaccine will be available for all individuals above the age of 18.
Hyderabad (Telangana)- based Indian biotechnology and biopharmaceutical company, Biological E Limited, will be the Indian partner for producing the vaccine.
Meanwhile, Biological E Limited is also planning to produce 75 million to 80 million doses of a new Covid-19 vaccine (Corbevax) in a month time.
The vaccine developers are Baylor College of Medicine in Houston and Dynavax Technologies Corp.