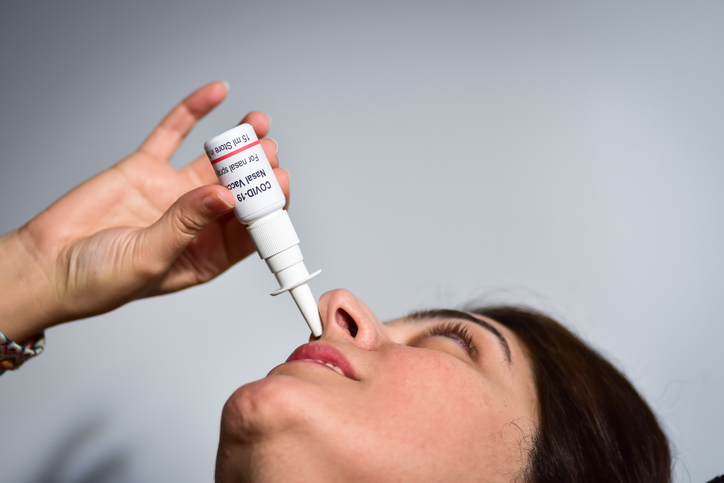
The Central Drugs Standard Control Organisation had approved the shot on November 28
New Delhi: The world’s first intranasal covid-19 shot, manufactured by Hyderabad-based Bharat Biotech, will be available on COWIN platform for people to take from next Friday, a report in The Tribune, Chandigarh, says
The introduction in the Indian covid inoculation plan comes in the wake of the surge in China and other countries.
The shot iNCOVACC (BBV154) had received approval from the Central Drugs Standard Control Organisation (CDSCO) under Restricted Use in Emergency Situation for ages 18 and above in India for heterologous booster doses on November 28.
Officials said the vaccine would be available at private centres at a nominal cost. The vaccine was earlier approved as a primary dose schedule in September.
iNCOVACC is a recombinant replication deficient adenovirus vectored vaccine with a pre-fusion stabilised SARS-CoV-2 spike protein. This vaccine candidate was evaluated in phases I, II and III clinical trials with successful results.
“iNCOVACC has been specifically formulated to allow intranasal delivery through nasal drops. The nasal delivery system has been designed and developed to be cost-effective in low- and middle-income countries. It was developed in partnership with Washington University, St Louis, which had designed and developed the recombinant adenoviral vectored construct and evaluated in preclinical studies for efficacy,” the company said in a statement.
Bharat Biotech.conducted product development related to preclinical safety evaluation, large-scale manufacturing scale up, formulation and delivery device development, including human clinical trials.
It was funded in part by the Government of India, through the Department of Biotechnology’s, Covid Suraksha Programme
Krishna Ella, Chairman of Bharat Biotech, said, “iNCOVACC is an intranasal vaccine for the primary 2-dose schedule, and heterologous booster dose. This is a great achievement for us and the global scientific community to enable nasal administration of covid vaccines. Despite the lack of demand for covid vaccines, we continued product development in intranasal vaccines to ensure that we are well-prepared with platform technologies for future infectious diseases.”
iNCOVACC is a recombinant replication deficient adenovirus vectored vaccine with a pre-fusion stabilised SARS-CoV-2 spike protein. This vaccine candidate was evaluated in phases I, II and III clinical trials with successful results.
Clinical trials were conducted to evaluate iNCOVACC as a primary dose schedule, and as heterologous booster dose for subjects who have previously received two doses of the two commonly administered covid vaccines in India.
Immunogenicity was evaluated through serum neutralising antibodies by PRNT assays and serum IgG’s through ELISA’s.
To evaluate the vaccine, taken through the intranasal route, IgAs were evaluated by ELISA in serum and saliva.
Evaluation was also carried out for ability of iNCOVACC to elicit responses against the ancestral and omicron variants.
Rajesh Gokhale, union biotechnology secretary, said, “The DCGI’s approval of Bharat Biotech’s intranasal vaccine iNCOVACC (BBV154) to be used as a heterologous booster dose against currently available covid vaccines is a moment of great pride for our country.”
******************************************************************
Readers
These are extraordinary times. All of us have to rely on high-impact, trustworthy journalism. And this is especially true of the Indian Diaspora. Members of the Indian community overseas cannot be fed with inaccurate news.
Pravasi Samwad is a venture that has no shareholders. It is the result of an impassioned initiative of a handful of Indian journalists spread around the world. We have taken the small step forward with the pledge to provide news with accuracy, free from political and commercial influence. Our aim is to keep you, our readers, informed about developments at ‘home’ and across the world that affect you.
Please help us to keep our journalism independent and free.
In these difficult times, to run a news website requires finances. While every contribution, big or small, will makes a difference, we request our readers to put us in touch with advertisers worldwide. It will be a great help.
For more information: pravasisamwad00@gmail.com